and we need keep that in mind.
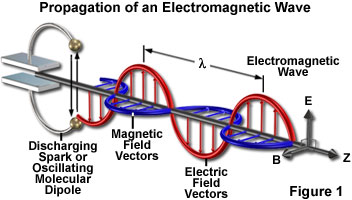
But we still don't really get it. Light is a form of electromagnetic energy. Here is the spectrum. All of these waves travel at the speed of light in a vacuum and act just like light. They have different wavelengths and frequencies but they travel like waves - electric and magnetic waves - and collide as particles.
From our equation,
we know that the higher frequency EM waves strike as particles with higher energy.
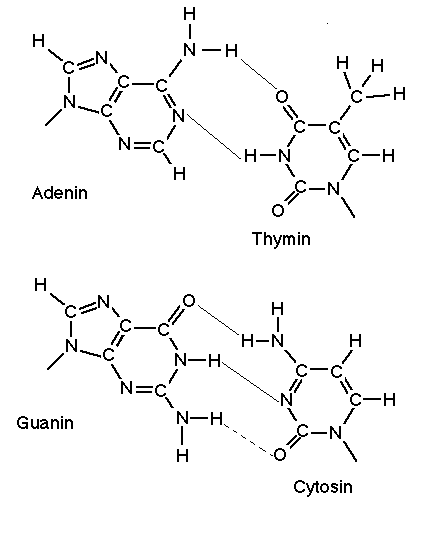
Now let's talk cancer. DNA must be changed in the cells of an organism in order for cancer to take hold.
Only waves above a certain threshold can strike DNA as particles with enough energy to knock electrons off and change the structure of DNA and perhaps cause cancer.
I say perhaps since this kind of thing happens on a regular basis and most cells repair themselves or self-destruct.
And NO. Microwaves cannot cause cancer any more than visible light can. Radio waves, cell phone signals, radar guns - none have enough energy when colliding as particles to do more than vibrate particles.
But the key to all this discussion is that LIGHT WAVES COLLIDE AS PARTICLES. And no studies have shown a real increase in cancer rate from cell phone or microwave oven use.
This video mentions frequency but misses that essential fact of the particle nature of waves when striking matter.
And here's Veritasium's weak and somewhat unclear video on the subject - that is what science is like - sometimes weak and unclear.
The key to this discussion is truly freaky. Electromagnetic waves act as particles when being generated - - and when colliding. That's why cell phones never cause cancer. Nor can microwaves or visible light.
So the next time Grandma or your friend tells you that cell phones - or whatever - can cause cancer, just tell her all about how those waves of radiation collide as particles and only the high energy particles like UV, X, or Gamma can cause changes in DNA chemical structure leading to possible cancer.
Or just smile and change the subject.
You've been told not to talk about religion or politics since it will lead to arguments. Add Freaky Fzx to the list - it just gets people angry. I've tried it.