BabyGirl buried her face in my shoulder and started screaming almost immediately - totally expected so I just walked away with her and sat down planning to cover her ears. As I was sitting, a gentleman from the Etna Fire Police offered me his truck. Not a bad idea so we sat in the front seat of his truck to watch the fireworks without all the sound after he cleared it of fast food wrappers.
Beautiful.
BUT
Here is the apparatus.
The year was 1905. 1897 saw the discovery of the electron and the Chocolate Chip Cookie Dough Model of the Atom reigned supreme. Rutherford had yet to discover the nucleus and the proton.
Einstein took center stage again with his explanation of another two decade unsolved problem in Physics.
Lets' step forward to a model of the atom that you are more familiar with for this explanation. Bohr didn't propose it until 1913 and we're still in 1905 so Einstein didn't have it but it'll help us.
When an electron is kicked out of an atom for some reason - heat or electricity or radiation - it leaves a space that must be filled.
Here is the emission.
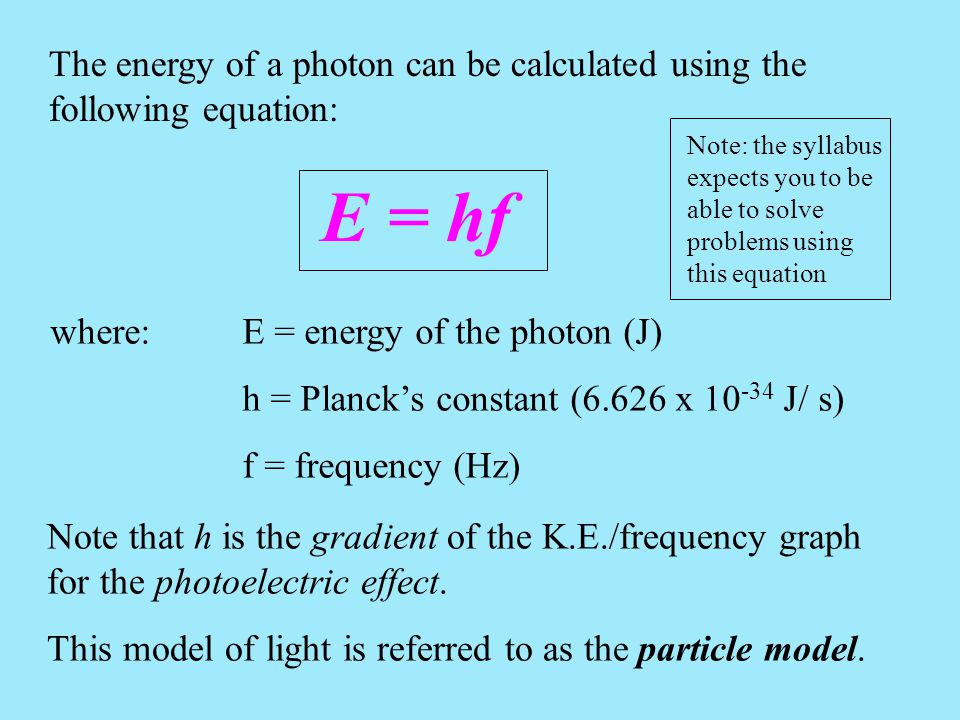
The frequency of the light emitted or absorbed is dependent upon this equation.
It works for absorption - the photoelectric effect - or emission - fireworks.
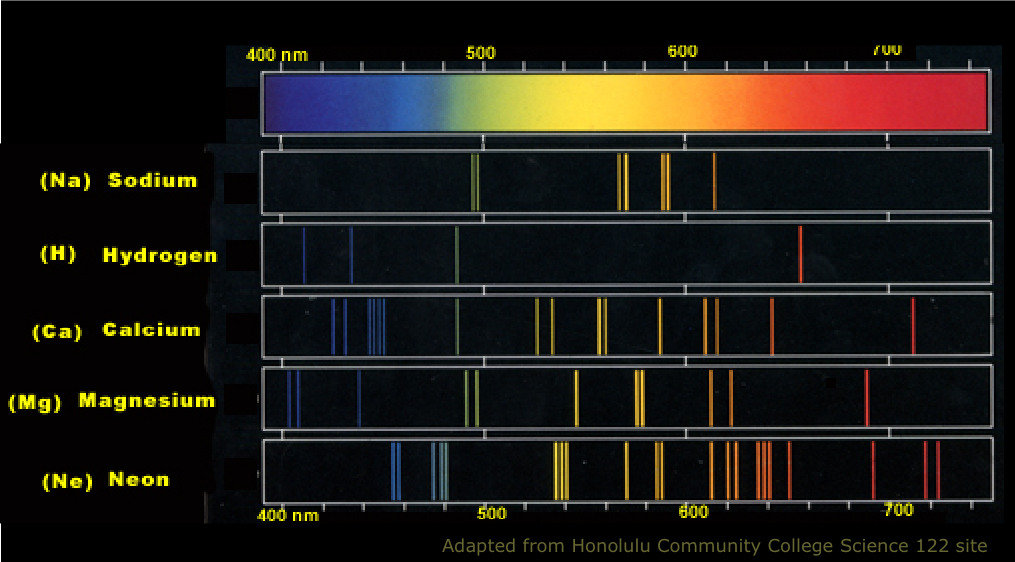
Every atom is different so every atom emits and absorbs different particles of light - different photons. More on that next time.
The crazy conclusion is that
But remember that this is what the atom really looks like.
It was Albert Einstein
again
in 1905
contributing major ideas to another field of study.
First it was Relativity and how different mass and spacetime are near the speed of light.
Then it was Quantum Theory and how what we know as waves can act as particles.
Those problems took an Einstein. It doesn't take an Einstein, however, to do well in this class.












No comments:
Post a Comment