Not quite the Law of Conservation of Matter but close. Almost the Law of Conservation of Life - Life begets life.
But even in my high school chemistry class we calculated the mass defect since particles lose mass when they form a nucleus. This is an example of mass loss and energy release - the energy source for our stars.
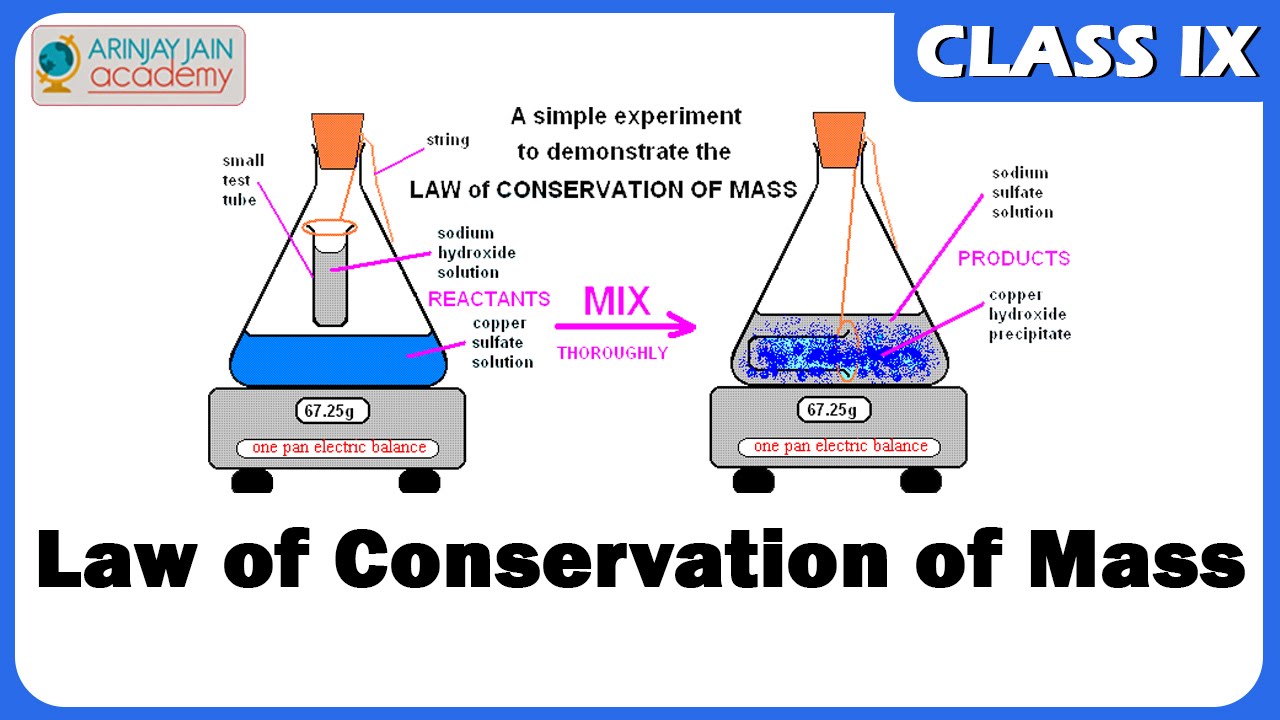
The above Law of Conservation of Mass was fundamental to a good understanding of Chemistry. Without it there would have been no chemistry.
And the Law of Conservation of Energy? Seriously important for the development of science
The idea that gravitational potential energy and heat from friction and energy of motion and nuclear bond energy are the same thing was a great leap forward.
The problem is that those conservation laws are not strictly true. What's the famous equation in the world?
Most people will interpret this as shown in the picture above but that's not a good translation. This is another Einstein idea and it's all about matter-energy equivalence.
Every time you eat or drive a car or use electricity from a coal, natural gas, or nuclear power plant - that's energy from matter. Chemical bond energy and nuclear bond energy manifest as mass.






No comments:
Post a Comment