Sharp Knife and Eagle Eyes
The name "atom" is, in fact, taken from the Greek word meaning "indivisible" but there was little good evidence either way until well after the 1500s and the beginnings of modern science.
Dalton and Lavoisier and Avogadro, in the early 1800's, began to understand the evidence for atoms - and molecules as combinations of atoms. A century later, after treating the atom as indivisible for 100 years, the atom started to split.
1897 - JJ Thomson discovered the electron and proposed my favorite cookie dough as a model.
The chips are the electrons but the dough is too solid.
Then Rutherford did his famous experiment where he fired BBs at a many-layered collection of mushy cookie dough balls and the BBs passed right through. He expected that result since he knew the BBs had high energy and the cookie dough balls weren't very solid. What Rutherford did not expect was that a small number of the BBs would bounce off the web of soft, warm, yummy cookie dough straight back at the source.
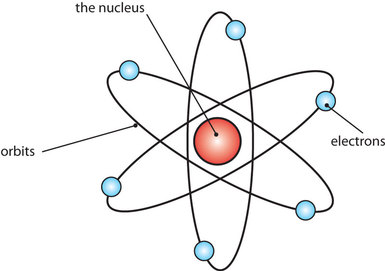
His conclusion was that the atom is not a ball of chocolate chip cookie dough and that's good since I'd never be able to maintain my boyish figure. Rutherford proposed the nuclear model of the atom - 1911.
Rutherford's atomic models
Bazinga! But just remember that all the models of the atom you have seen are total crap.
They get some things right, but if the scale were correct, this would be the model of the atom:
The best scale model of the atom
We split the atom more than a century ago. Whatever. Splitting the nucleus is a much bigger deal.




No comments:
Post a Comment