As with everything else in physics, the reality is much more complicated.
The idea that protons and neutrons are composed of smaller particles was first proposed in the early 1960s to explain observed phenomena that contemporary theory could not. "Quarks," (from James Joyce's Finnegans Wake - "three quarks for muster mark,") were initially treated as mathematical constructs and not as real particles but that soon changed.
In an experiment similar to Rutherford's Gold Foil Scattering Experiment in the late 1960s,
researchers fired electrons at protons and observed the resulting electron paths with a degree of precision impossible just a few years before. Just like Rutherford's nucleus backscattering alpha particles, the electrons ricocheted off 3 separate hard objects inside the proton, proving that quarks are real objects and not just a math trick.
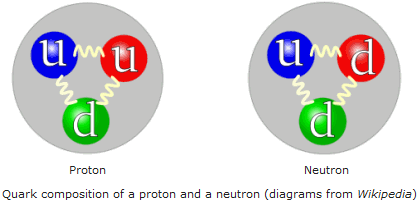
The proton consists of two "UP" quarks and one "DOWN" quark while the neutron is two downs and an up.
It took scientists a long time to find all this because the atom and its parts are so unimaginably small. Check out the dimensions in this picture.
+2/3 and -1/3 of the "fundamental charge" add to give the proton and neutron their characteristic charges. Here's a summary of some of the fundamental particles of the universe
and an interview with the scientist who proposed the existence of quarks.
So the atom, named "unsplittable" in Greek, has far more components than we ever thought possible.




No comments:
Post a Comment